










 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |


 Silicon Valley's dot-com era may be giving way to the watt-com era.
Silicon Valley's dot-com era may be giving way to the watt-com era.
Out of the ashes of the Internet bust, many technology veterans have re-grouped and found a new mission in alternative energy: developing wind power, solar panels, ethanol plants and hydrogen-powered cars.
It is no secret that venture capitalists have begun pouring billions into energy-related start-ups. But that interest is now spilling over to many others in Silicon Valley—lawyers, accountants, recruiters and publicists, all developing energy-oriented practices to cater to the cause.
The best and the brightest from leading business schools are pelting energy start-ups with resumes. And, of course, there are entrepreneurs from all backgrounds—but especially former dot-commers—who express a sense of wonder and purpose at the thought of transforming the $1 trillion domestic energy market while saving the planet.
The participants here say their whole approach to building new companies and industries is easily transferable to the energy world. But critics wonder whether this is just an echo of the misguided optimism of the Internet boom. And even those most involved in the trend say the size of the market opportunity in energy is matched by immense hurdles.
"There are real business models and real products to be sold—established markets and growing economics," said George Basile, who has a doctorate in biophysics from the University of California, Berkeley, and specialises in energy issues.
1. Oil
 Platforms from Construction to Decommission
Platforms from Construction to Decommission
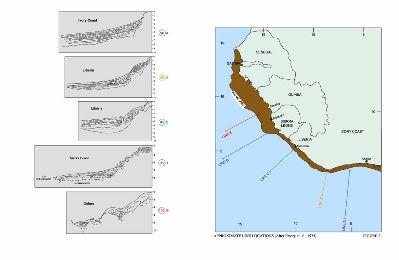 Oil Prospection and extraction
Oil Prospection and extraction
 2. Diesel out of organic waste through catalytic depolimerisation
2. Diesel out of organic waste through catalytic depolimerisation
4. Energy for the Cities
5. Alternative Energies
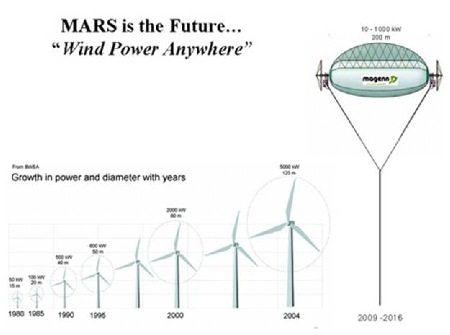
The Advantages of MARS over Conventional Wind Turbines are:
1. low cost electricity - under 15 cents per kWh
2. bird and bat friendly
3. lower noise
4. wide range of wind speeds - 2 to more than 28 meters/second
5. higher altitudes - from 200 to 1,000 feet above ground level are possible without expensive towers or cranes
6. fewer limits on placement location - coast line placement is not necessary
7. ability to install closer to the power grid
8. mobile
9. ideal for off grid applications or where power is not reliable.
Initial MARS Target Markets include:
1. Developing and island nations where infrastructure is limited or non existent
2. Rapid deployment (to include airdrop) to disaster areas for power to emergency and medical equipment, water pumps, and relief efforts (ex. Katrina, Tsunami)
3. Off grid for cottages and remote uses such as cell towers and exploration equipment)
4. and disaster and military applications..
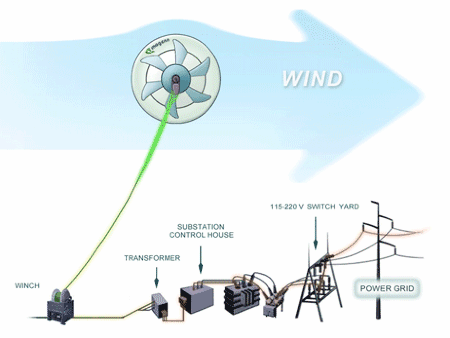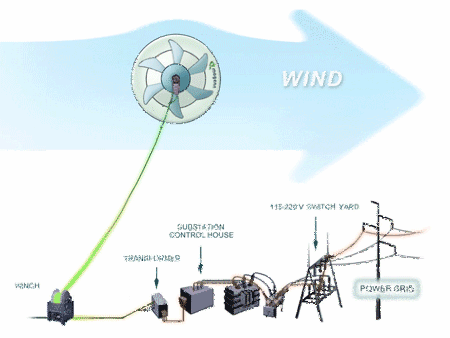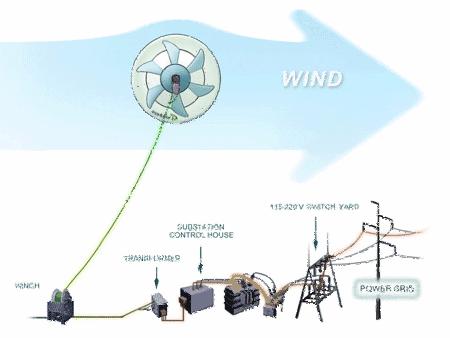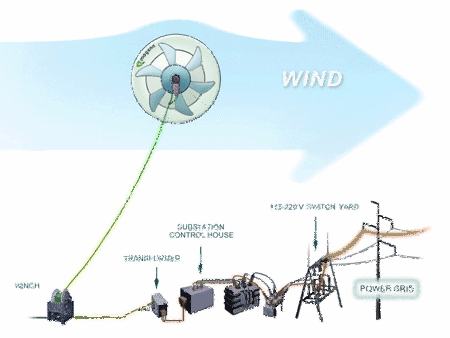
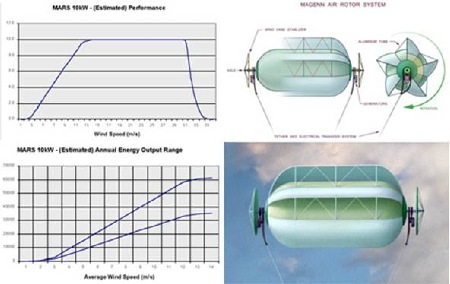
| Magenn Power Product from NaMPET | Model 10kW |
| Rated Power | 10,000 Watts |
| Size (Diameter x Length) | 30 feet by 60 feet |
| Shipping Weight | Under 3,000 lbs - depending on tether length |
| Volume of Helium | 33,000 cubic feet (approx.) |
| Tether Height | 400 ft standard - up to 1,000 ft optional tether length, in increments of 100 feet |
| Start-up Wind Speed | 2.0 m/sec - 4.48 mph |
| Cut-in Wind Speed | 3.0 m/sec - 6.7 mph |
| Rated Wind Speed | 12.0 m/sec - 26.8 mph |
| Cut-out Wind Speed | 25.0 m/sec - 53.7 mph |
| Maximum Wind Speed | 28.0 m/sec - 62.6 mph |
| Temperature Range | -40ºC /-40ºF to +45ºC/+113ºF |
| Generators | 2 x 5 kW |
| Output Form | Various Options Available: 120 VAC 60Hz - 240 VAC 50 Hz - Regulated DC 12-600V |
| Warranty | Up to 5 Years |
| Life Cycle | 10 - 15 Years |

6. Reduction of Energy consumption in Construction through Aerogels

Aerogel is a synthetic porous ultralight material derived from a gel, in which the liquid component of the gel has been replaced with a gas. The result is a solid with extremely low density and low thermal conductivity.
Aerogel was first created by Samuel Stephens Kistler in
1931, as a result of a bet with Charles Learned over who could replace
the liquid in "jellies" with gas without causing shrinkage.
Aerogels are produced by extracting the liquid component of a gel through supercritical drying. This allows the liquid to be slowly dried off without causing the solid matrix in the gel to collapse from capillary action, as would happen with conventional evaporation. The first aerogels were produced from silica gels. Kistler's later work involved aerogels based on alumina, chromia and tin dioxide. Carbon aerogels were first developed in the late 1980s.
Properties
 Aerogel has excellent insulating properties, and the flower is protected from the flame.
Aerogel has excellent insulating properties, and the flower is protected from the flame.
Despite their name, aerogels are solid, rigid, and dry
materials that do not resemble a gel in their physical properties:
The
name comes from the fact that they are made from gels. Pressing softly
on an aerogel typically does not leave even a minor mark; pressing more
firmly will leave a permanent depression. Pressing extremely firmly will
cause a catastrophic breakdown in the sparse structure, causing it to
shatter like glass – a property known as friability;
although more modern variations do not suffer from this. Despite the
fact that it is prone to shattering, it is very strong structurally. Its
impressive load bearing abilities are due to the dendritic microstructure, in which spherical particles of average size (2–5 nm) are fused together into clusters. These clusters form a three-dimensional highly porous structure of almost fractal chains,
with pores just under 100 nm. The average size and density of the pores
can be controlled during the manufacturing process.
Aerogels are good thermal insulators because they almost nullify two of the three methods of heat transfer (convection, conduction, and radiation). They are good conductive insulators
because they are composed almost entirely from a gas, and gases are
very poor heat conductors. Silica aerogel is especially good because
silica is also a poor conductor of heat (a metallic aerogel, on the
other hand, would be less effective). They are good convective inhibitors
because air cannot circulate through the lattice. Aerogels are poor
radiative insulators because infrared radiation (which transfers heat)
passes right through silica aerogel.
Owing to its hygroscopic nature, aerogel feels dry and acts as a strong desiccant. Persons handling aerogel for extended periods should wear gloves to prevent the appearance of dry brittle spots on their skin.
The slight color it does have is due to Rayleigh scattering of the shorter wavelengths of visible light by
the nano-sized dendritic structure. This causes it to appear smoky blue
against dark backgrounds and yellowish against bright backgrounds.
Aerogels by themselves are hydrophilic, but chemical treatment can make them hydrophobic.
If they absorb moisture they usually suffer a structural change, such
as contraction, and deteriorate, but degradation can be prevented by
making them hydrophobic. Aerogels with hydrophobic interiors are less
susceptible to degradation than aerogels with only an outer hydrophobic
layer, even if a crack penetrates the surface. Hydrophobic treatment
facilitates processing because it allows the use of a water jet cutter.
Knudsen effect
Aerogels may have a thermal conductivity smaller than the gas they contain. This is caused by the Knudsen effect.
Knudsen effect is the reduction of thermal conductivity in gases when
the size of the cavity encompassing the gas becomes comparable to the mean free path.
Effectively, the cavity restricts the movement of the gas particles,
decreasing the thermal conductivity in addition to eliminating
convection. For example, thermal conductivity of air is about 25 mW/m·K
at STP and in a large container, but decreases to about 5 mW/m·K in a
pore 30 nanometers in diameter.
Materials
A 2.5 kg brick is supported by a piece of aerogel with a mass of only 2 grams.
Silica
Silica aerogel is the most common type of aerogel, and the most extensively studied and used. It is silica-based, derived from silica gel.
The lowest-density silica nanofoam weighs 1,000 g/m3, which is the
evacuated version of the record-aerogel of 1,900 g/m3. The density of air is 1,200 g/m3 (at 20 °C and 1 atm). As of 2013, aerographene had a lower density at 160 g/m3, or 0.13 times the density of air at room temperature.
It has remarkable thermal insulative properties, having an extremely low thermal conductivity: from 0.03 W/m·K in atmospheric pressure down to 0.004 W/m·K in modest vacuum, which correspond to R-values of
14 to 105 (US customary) or 3.0 to 22.2 (metric) for 3.5 in (89 mm)
thickness. For comparison, typical wall insulation is 13 (US Customary)
or 2.7 (metric) for the same thickness. Its melting point is 1,473 K (1,200 °C; 2,192 °F).
Until 2011, silica aerogel held 15 entries in Guinness World Records for
material properties, including best insulator and lowest-density solid,
though it was ousted from the latter title by the even lighter
materials aerographite in 2012 and then graphene aerogel in 2013.
Carbon
Carbon aerogels are composed of particles with sizes in the nanometer range, covalently bonded together. They have very high porosity (over
50%, with pore diameter under 100 nm) and surface areas ranging between
400–1,000 m2/g. They are often manufactured as composite paper:
non-woven paper made of carbon fibers, impregnated with resorcinol–formaldehyde aerogel, and pyrolyzed.
Depending on the density, carbon aerogels may be electrically
conductive, making composite aerogel paper useful for electrodes in capacitors or deionization electrodes. Due to their extremely high surface area, carbon aerogels are used to create supercapacitors, with values ranging up to thousands of faradsbased
on a capacitance of 104 F/g and 77 F/cm3. Carbon aerogels are also
extremely "black" in the infrared spectrum, reflecting only 0.3% of
radiation between 250 nm and 14.3 µm, making them efficient for solar energy collectors.
The term "aerogel" to describe airy masses of carbon nanotubes produced through certain chemical vapor deposition techniques is incorrect. Such materials can be spun into fibers with strength greater than Kevlar,
and unique electrical properties. These materials are not aerogels,
however, since they do not have a monolithic internal structure and do
not have the regular pore structure characteristic of aerogels.
Alumina
Aerogels made with aluminium oxide are
known as alumina aerogels. These aerogels are used as catalysts,
especially when "doped" with a metal other than aluminium. Nickel–alumina aerogel is the most common combination. Alumina aerogels are also being considered by NASA for capturing hypervelocity particles; a formulation doped with gadolinium and terbium could fluoresce at the particle impact site, with the amount of fluorescence dependent on impact energy.
Other
SEAgel is a material similar to organic aerogel, made of agar. Spaceloft is a fabric form of aerogel.
Chalcogel is an aerogel made of chalcogens (the
column of elements on the periodic table beginning with oxygen) such as
sulfur, selenium and other elements. Metals less expensive than
platinum have been used in its creation.
Aerogels made of cadmium selenide quantum dots in a porous 3-D network have been developed for use in the semiconductor industry.
Aerogel performance may be augmented for a specific application by the addition of dopants, reinforcing structures and hybridizing compounds.

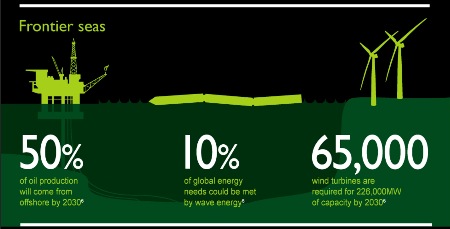
Neuer Absatz


















 3.
3.